Boyle's Law Data Table
The core is placed in a sample chamber of known volume. N R and P are constant.
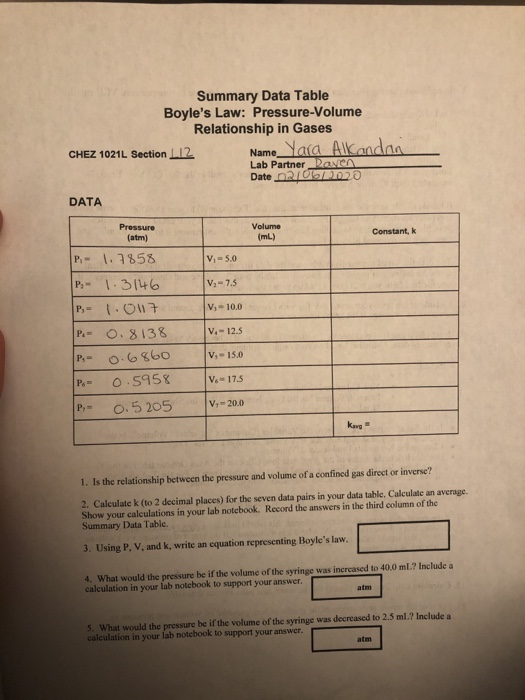
Solved Summary Data Table Boyle S Law Pressure Volume Chegg Com
Boyles law also referred to as the BoyleMariotte law or Mariottes law especially in France is an experimental gas law that describes how the pressure of a gas tends to decrease as the volume of the container increases.
. Scientific calculator for chemists - can be used as a common scientific calculator sin cos log power root memory but also contains a molecular weight calculator and tables with various physical and chemical constants. Boyles law - The formula used when dealing with an isothermal process a process where the temperature does not change. The Kohlrausch law and its applications are crucial in the study of dilute liquids as well as electrochemical cells.
Fluorine is an element with atomic number 9 and symbol F. Mercury was added to the tube trapping a fixed quantity of air in the short sealed end of the tube. Click or tap and drag across the graph to select a region.
In 1662 Robert Boyle performed a series of experiments employing a J-shaped glass tube which was sealed on one end. The data table lists the dry wet. Link to BBO Data 132nd Irish Intermediate Pairs Championship Thursday 25th August 22 Tables Congratulations to Kat Hynes and Josephine Brady Drogheda and Navan 2nd Renee and Pat OSullivan Sligo and 3rd Ann Caulfield and Raymond Hearty Clontarf Leaderboard and Board Details Link to BBO Data Congratulations to Sonya Hillis and Huey Daly who.
Law of Multiple Proportions Click Here for Sample Questions The Law of Multiple Proportions states that If two elements combine to form more than one compound between them the mass ratios of the second element which combine with a fixed mass of the first element will always be the ratios of small whole numbers. The notion that the Fair Share Act does not apply when an injured party plaintiff is an innocent victim of the negligence of another seems to. The two chambers are then connected.
The helium pycnometer makes use of Boyles Law P 1 V 1 P 2 V 2 and helium gas which quickly penetrates small pores and is nonreactive to determine the solid portion of a sample. The statistics and computer science departments have joined forces to offer a minor in data science. Then the volume of gas was carefully measured as additional mercury was added to the.
Calculator - the best online scientific calculator. Analyze data to support the claim that the combined gas law describes the relationships among volume. In general Tt T A T H-T Ae-kt where Tt is the Temperature at time t T A is the Ambient temperature or temp of surroundings T H is the temperature of the hot object k is the positive constant and t is the time.
The Kohlrausch Law of Independent Migration is another name for this law. N R and P are constant. P 1 V 1 T 1 P 2 V 2 T 2 nR.
Partial pressure of the gas P 4 atm Henrys law constant k 15 L atmmol Using the equation of. Boyles Law HS-PS1-10Use evidence to support claims regarding the formation properties and behaviors of solutions at bulk scales. Use the previous formula and the constant from Table 1 to calculate the temperature at which a solution of 50 grams of sucrose C 12 H 22 O 11 in 400 grams of water will freeze.
A reference chamber also of known volume is pressurized. P 1 V 1 P 2 V 2 CharlesGay-Lussac Law P is constant. Calculate the concentration of a helium gas dissolved in water if the partial pressure of the gas is 4 atm and the value of henrys law constant k 15 L atmmol.
PV is constant. Select a region for additional data analysis. Courses such as Applied Multivariate Statistics and Data Mining STAT 530 and Big Data Analytics STAT.
Concentration of a dissolved gas C. Apply a Curve Fit to a Selected Region. For a given amount of matter therefore nR is constant which gives the Ideal Gas Law.
Analyze a selection of your data. Methods to Apply Newtons Law of Cooling. Zeroeth Law of Thermodynamics - Two.
This law was proposed by Dalton in. The molecular weight of sucrose is 121201 22101 111600 34234 gmole. Among other essential uses this law is utilised to determine the limiting conductivity of a weak electrolyte.
Laws of Thermodynamics. V 1 T 1 V 2 T 2 Ideal Gas Law. Data science and big data analytics are hot areas these days as companies are realizing the value of learning from large data sets.
If needed drag the boundary lines to resize the region. Boyles Law T is constant. The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the.
Charless law - The formula used when dealing with an isobaric process a process where the temperature does not change. Boyles law was perhaps the first expression of an equation of state. Henrys Law Practice Problems.
Know the Uses of Fluorine Chemical Properties of Fluorine Atomic Mass of Fluorine more at BYJUS. What is Kohlrauschs Law. R is the ideal gas constant R 83145 JmolK.
A modern statement of Boyles law is. It is assumed that a constant rate of cooling which is equal to the rate of cooling related to the average. General gas equation Charles law Gay-Lussacs law Boyles.
To the relationships among the variables of the combined gas law not the gas law names ie. Table 1 gives data for several common solvents. Click or tap the.
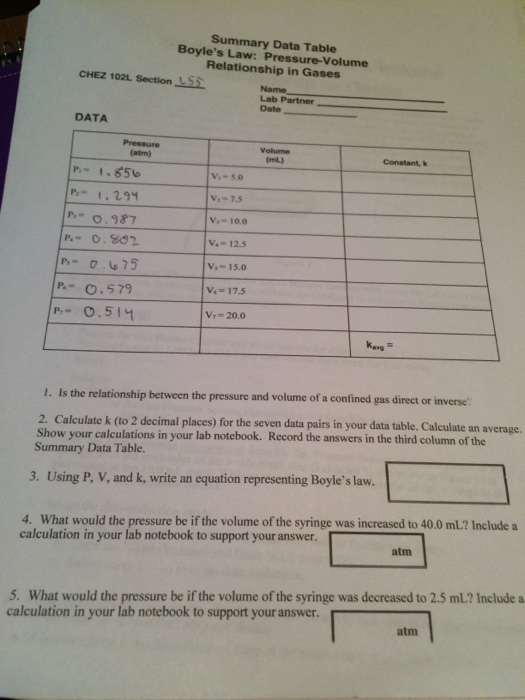
Solved Summary Data Table Boyle S Law Pressure Volume Chegg Com
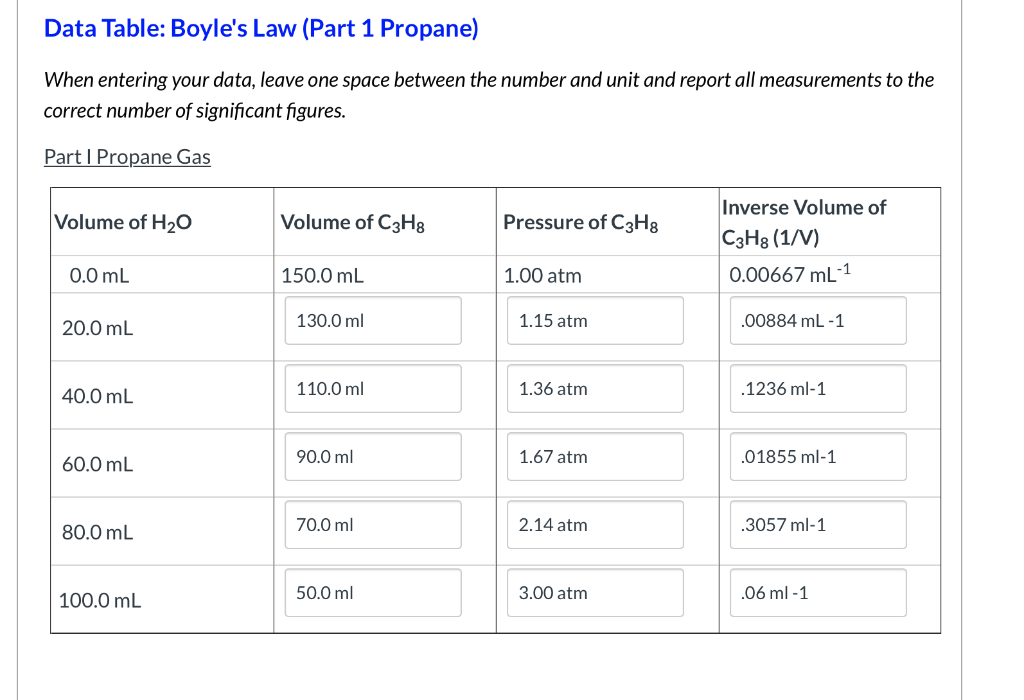
Data Table Boyle S Law Part 1 Propane When Chegg Com

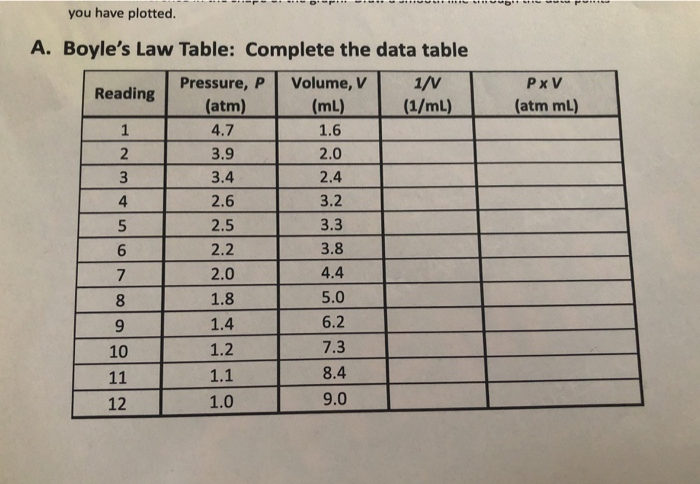
Solved You Have Plotted Pxv Atm Ml 4 A Boyle S Law Chegg Com
No comments for "Boyle's Law Data Table"
Post a Comment